TMJ Safety Device Cannula Kits
ANVISA Registration: 80455639010 | 80455639013 | 80455639014
The TMJ Sutures Safety Device Cannula Kit Traumec includes instruments that safely access the portals inside the Temporomandibular Joint by temporarily coupling the safety device (insertion stop) to permanent and disposable trocar cannulas, enabling controlled depth of access to the capsule as planned, preventing injuries, perforations, and undesired tissue damage.

INDICATIONS
The TMJ Sutures Safety Device Cannula Kits are indicated for minimally invasive surgical procedures, such as video-assisted procedures, primarily small joint arthroscopies:
- TMJ Discopexy.
- Disc arthroscopy.
- Disc myotomy.
- Disc rupture.
- Intra-articular pain.
- Removal of adhesions and synovial disk adherences.
- Diagnostic.
- Biopsies.
- Synovitis.
- TMJ disc stabilization suture, specifically the condylar process of the mandible with the temporal bone.
FEATURES AND BENEFITS
- The TMJ Sutures Safety Device Cannula Kits include the Interchangeable Stop Safety Device 2.0 / 3.0, designed to limit the cannula insertion with the obturator, preventing damage to adjacent tissues.
- Made of Stainless Steel UNS S30400 ASTM F899, Polypropylene (PP), Silicone, Photosensitive Resin, and Low-Density Polyethylene (LDPE) Tubular Plastic.
- Sterilized product by Ethylene Oxide – ETO.
- Single-use.
INSTRUCTIONS FOR USE
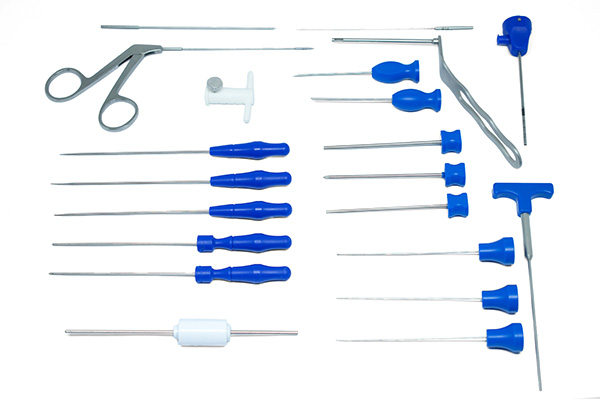
TMJ Sutures All Tec Safety Device Cannula Kit (Code PA.02.03.4420) consists of the following components:
- PA.02.03.4400 - Safety Device Cannula - 01
- PA.02.03.4412 - Safety Device Obturator - 01
- PA.02.03.4413 - Safety Device Trocar - 01
- PA.02.03.2888 - Banana Knife Via Cannula - 01
- PA.02.03.2889 - Rasp Hook Via Cannula - 01
- PA.02.03.2890 - Minescotomy Via Cannula - 01
- PA.02.03.2891 - Arthroscopy Clamp Via Cannula - 01
- PA.02.03.2895 - Shaver via Cannula Innovation - 01
- PA.02.03.4407 - Interchangeable Stop Safety Device 2.0 / 3.0 - 01
- PA.02.03.2894 – Sheath – 01
- PA.02.14.0009 - Straight Cannula
- PA.02.14.0014 - Curved Cannula
- PA.02.14.0011 - Obturator
- PA.02.14.0012 - Perforator
- PA.02.14.0010 - Thread Manipulator
- PA.02.14.0013 - Suture Button - 02
- PA.02.03.4401 - Smart Knot Drill DIAM. 1.0 ATM - 01
- PA.02.03.4402 - Smart Knot Guide ATM - 01
- PA.02.03.4403 - Smart Knot Obturator ATM - 01
- PA.02.03.4404 - Smart Knot Reduction Cannula 1.0 ATM - 01
- PA.02.03.4406 - Knot Drill DIAM. 1.5 ATM - 01
- PA.02.03.4411 - Knot Reduction Cannula 1.5 ATM - 01
- PA.02.03.4405 - Smart Knot ATM Pusher - 01

The TMJ Plain Safety Device Cannula Kit (Code PA.02.03.4422) consists of the following components:
- PA.02.03.4400 - Safety Device Cannula - 01
- PA.02.03.4412 - Safety Device Obturator - 01
- PA.02.03.4413 - Safety Device Trocar - 01
- PA.02.03.2888 - Banana Knife Via Cannula - 01
- PA.02.03.2889 - Rasp Hook Via Cannula - 01
- PA.02.03.2890 - Minescotomy Via Cannula - 01
- PA.02.03.4423 - Planer Via Cannula - 01
- PA.02.03.4424 - Curette Via Cannula - 01
- PA.02.03.4407 - Interchangeable Stop Safety Device 2.0 / 3.0 - 01
- PA.02.03.2894 – Sheath – 01
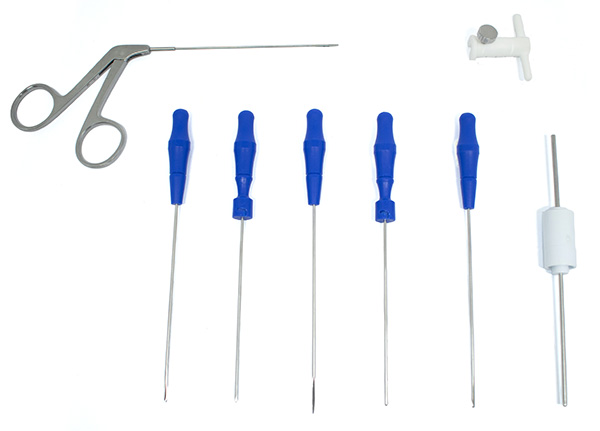
The TMJ High Safety Device Cannula Kit (Code PA.02.03.4421) consists of:
- PA.02.03.4400 - Safety Device Cannula - 01
- PA.02.03.4412 - Safety Device Obturator - 01
- PA.02.03.4413 - Safety Device Trocar - 01
- PA.02.03.2888 - Banana Knife Via Cannula - 01
- PA.02.03.2889 - Rasp Hook Via Cannula - 01
- PA.02.03.2890 - Minescotomy Via Cannula - 01
- PA.02.03.2891 - Arthroscopy Clamp Via Cannula - 01
- PA.02.03.2895 - Shaver via Cannula Innovation - 01
- PA.02.03.4407 - Interchangeable Stop Safety Device 2.0 / 3.0 - 01
- PA.02.03.2894 – Sheath – 01


