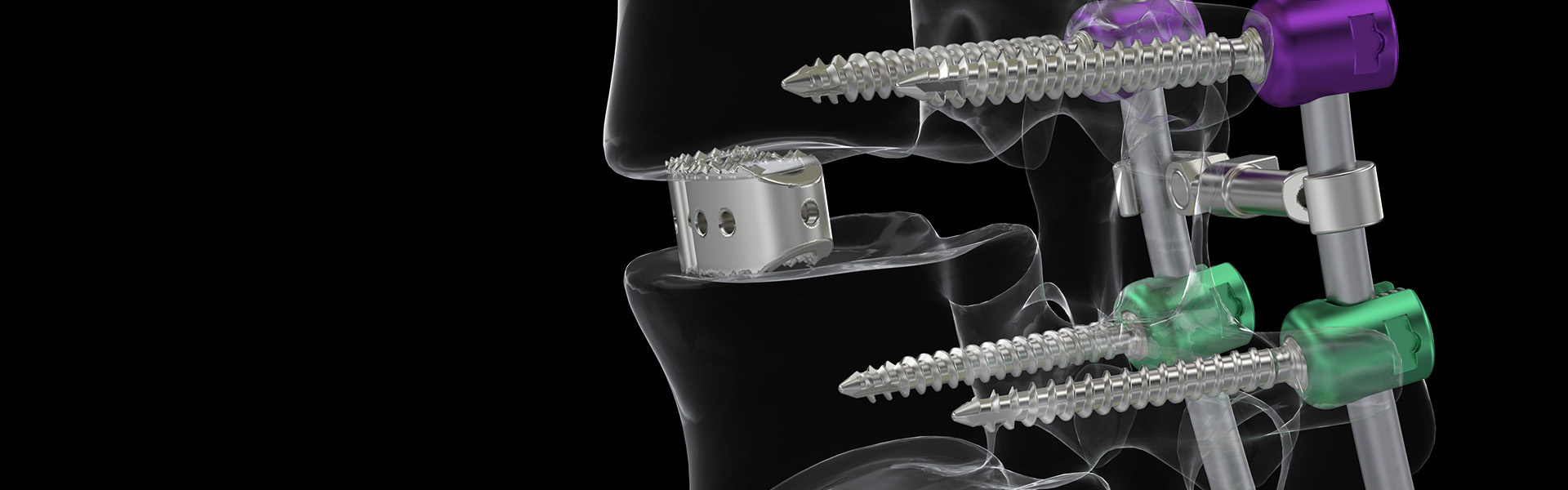
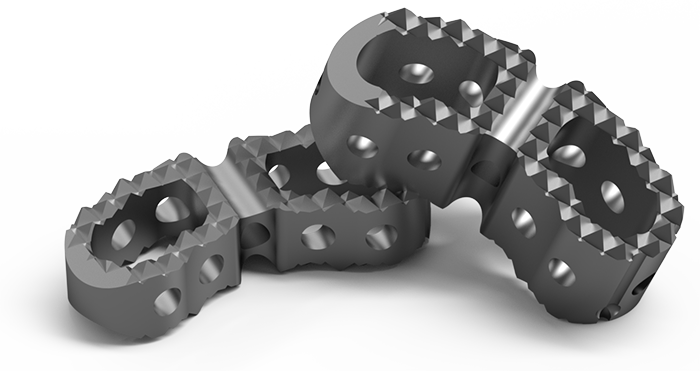
Lumbar Interbody Spacer
ANVISA Registration: 80455630005 | 80455630006

FEATURES AND BENEFITS
- Manufactured in Ti6AL4V alloy according to ASTM F136 specifications;
- Available in dimensions 30 x 13mm and varied heights: 07mm | 08mm | 09mm | 10mm | 11mm | 12mm | 13mm;
- The implants have a curved geometric shape, similar to an arc, for better accommodation in the vertebral body, with through slots and upper and lower ends formed by a serrated surface designed to improve implant fixation, preventing its migration.
- Central cavity for graft insertion;
- Serrated surface for primary fixation and reduced risk of spacer migration;
- Chamfered tip profile, facilitating implant insertion;
- Instruments with ergonomic and rubber-coated handles, produced with high quality;
INDICATIONS
- Posterior lumbar interbody fusion in cases of trauma, fracture, and degenerative diseases.
INSTRUCTIONS FOR USE