Orthognathic Module
ANVISA Registration: 80455630030 | 80455630104
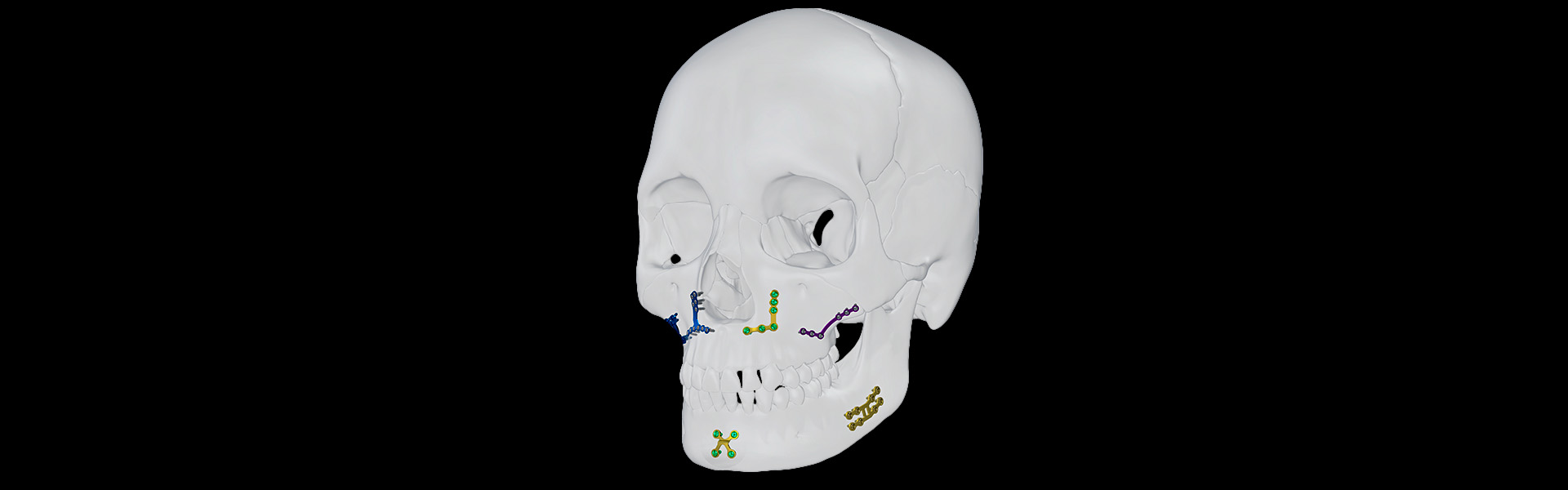
The Orthognathic Module offers a variety of solutions for treating all types of facial dento-skeletal deformities. It is indicated for osteosynthesis in orthognathic surgery, including the maxilla, mandible, and chin, offering a diversity of plates with differentiated geometries from the 1.5 and 2.0 mm systems that facilitate osteotomy fixation.

FEATURES AND BENEFITS
- Screws in self-tapping and self-drilling options, manufactured in ASTM F-136 Titanium (TI 6AL 4V ELI) with proven efficiency in penetration and mechanical resistance, providing safety and speed in the surgical procedure;
- Innovative design plates with low profile, easy modeling, and adaptation, manufactured in ASTM F-67 Titanium (Grade 2 pure TI);
- Self-drilling locking screws from 6 mm to 12 mm;
- Drills Ø 1.1 and Ø 1.5 mm with stops at 5 mm and 20 mm;
- Cross Drive adapters 1.5 and 2.0 for contra-angle;
- System 1.5 plates with a thickness of 0.6 mm;
- Special plates with a thickness of 0.5 mm;
- System 2.0 plates with thicknesses of 0.9 mm and 1.0 mm;
INDICATIONS
- Le Fort I, II, and III osteotomy;
- Bilateral sagittal osteotomy of the mandibular branch;
- Mentoplasty.
INSTRUCTIONS FOR USE