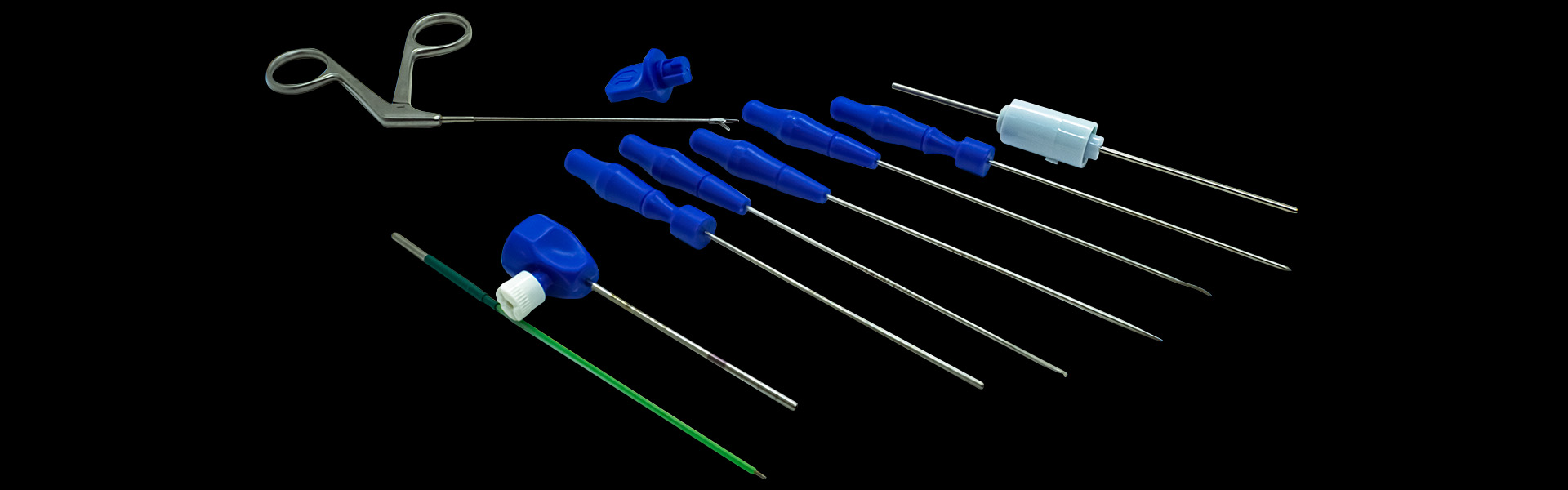
Cannulated Kit New Innovation
ANVISA Registration: 80455630058 | 80455630023
The CANNULATED KIT NEW INNOVATION is indicated for use in minimally invasive surgical procedures, such as video-assisted procedures (arthroscopy of small joints).

Composition
- Innovation Cannula
- Obturator via Cannula
- Trocar Cannula
- Banana Knife via Cannula
- Scraper Hook via Cannula
- Meniscotome via Cannula
- Arthroscopy Forceps via Cannula
- Plug
- Cannula cap
- Sleeve
- Shaver via Cannula Innovation
- Straight Dissector 108.5 X 45° X 3 Mm N
FEATURES AND BENEFITS
- Components made of Stainless Steel UNS S30400 and UNS S42000, Polypropylene (PP), Silicone, Low-Density Polyethylene (LDPE), Flexible PVC, and ABS.
- Sterilized by ETO method;
- Single-use product.
INDICATIONS
- Tissue manipulations;
- Adhesion reduction.
INSTRUCTIONS FOR USE