ATM Standard Prosthesis
ANVISA Registration: 80455630115
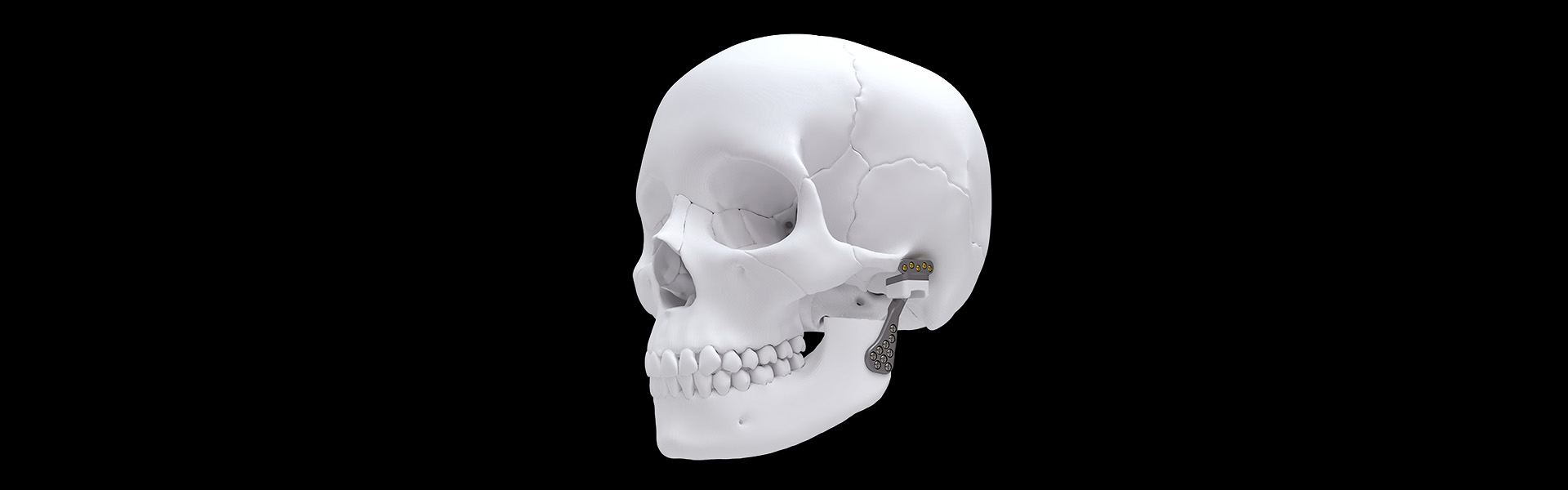
The ATM Total Arthroplasty System – Traumec enables the total replacement of the Temporomandibular Joint (ATM), providing immediate functionality for the affected mandibular branch in patients with mandibular fractures, mandibular deformities, or any disease compromising the Temporomandibular Joint.

FEATURES AND BENEFITS
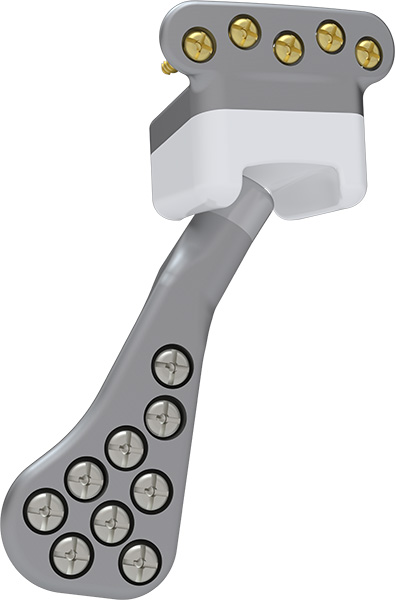
- Manufactured from 4 raw materials: Titanium Alloy Ti6Al4V ASTM F136; Pure Titanium ASTM F67; Cobalt Chromium Alloy (Co-Cr ASTM F1537); and Ultra-High Molecular Weight Polyethylene (UHWMPE ASTM F648) due to their properties making these materials ideal for implantable medical devices. Their main properties are biocompatibility and mechanical resistance.
- Fixation mechanisms of the mandibular component head and the polyethylene fossa to the underlying titanium structures prevent gaps, rotations, and displacements, delivering a biomechanically reliable set;
- Significant improvement in pain, speech, and eating function, mouth opening;
- Immediate function recovery;
- Satisfactory aesthetic results;
- Product fixation is performed with cortical crossdrive screws Ø2.0mm (or Ø2.3mm in emergency fixation cases) for the cranial component and screws Ø2.4mm (or Ø2.7mm in emergency fixation cases) for the mandibular component (ancillary components of this process, registered and sold separately);
- Three fossa sizes (S, M, and L) for both right and left sides.
- Five condylar plate sizes: three sizes (S, M, L) for the standard model and two sizes (S, M) for the SLIM model.
INDICATIONS
- Osteoarthritis, traumatic arthritis, rheumatoid arthritis;
- Ankylosis, with excessive heterotopic bone formation;
- Revision procedures where other treatments have failed (e.g., alloplastic reconstruction and autogenous grafts);
- Osteonecrosis;
- Fractures;
- Significant functional deformities;
- Benign neoplasms;
- Post-tumor excision reconstructions;
- Degenerated or resorbed joints with severe anatomical discrepancies;
- Developmental anomalies.
INSTRUCTIONS FOR USE